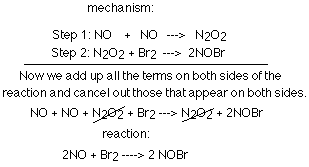Chpt 12 - Chemical Kinetics Reaction Rates Rate Laws Reaction Mechanisms Collision Theory Catalysis HW set1: Chpt 12 - pg , # 22, 23, 28 Due Jan. - ppt download

Reaction mechanism Most reactions occur in a series of steps. Most you don't see. The reaction mechanism is these series of steps. - ppt download

The rate constant for the decompoistion of a certain reaction is described by the equation: log k(s^(-1)) = 14 - (1.25 xx 10^(4) K)/(T) A two-step mechanism has been suggested for the
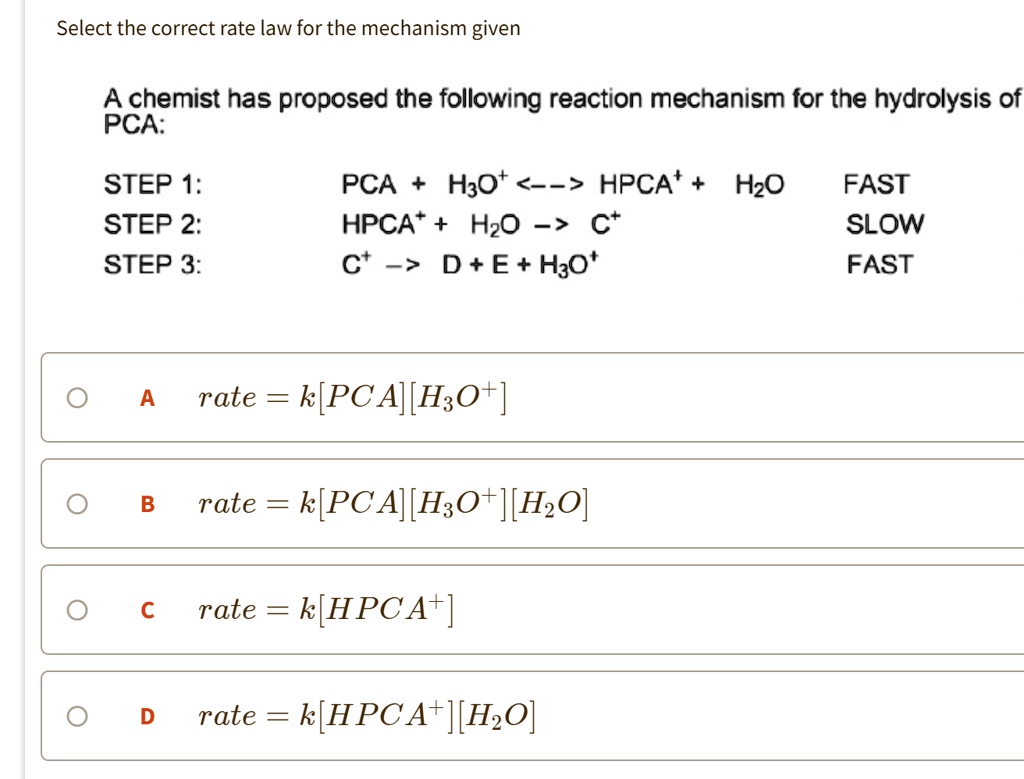
SOLVED:Select the correct rate law for the mechanism given A chemist has proposed the following reaction mechanism for the hydrolysis of PCA: STEP 1: STEP 2: STEP 3: PCA HzOt . 4->
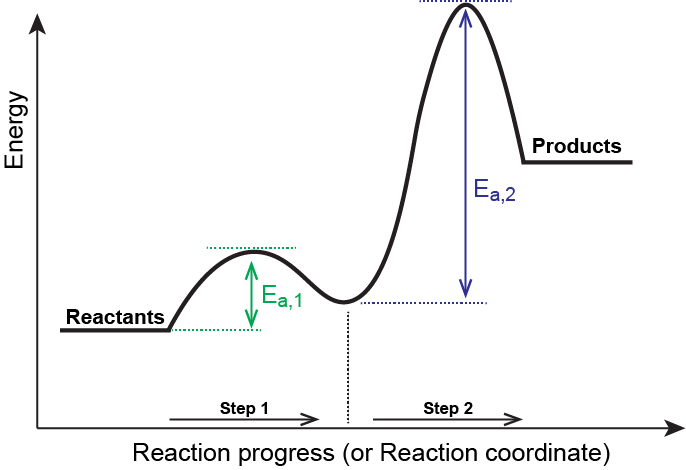
M13Q10: Mechanisms and Multistep Reactions; Reaction Profiles; Rate Limiting Steps – Chem 103/104 Resource Book
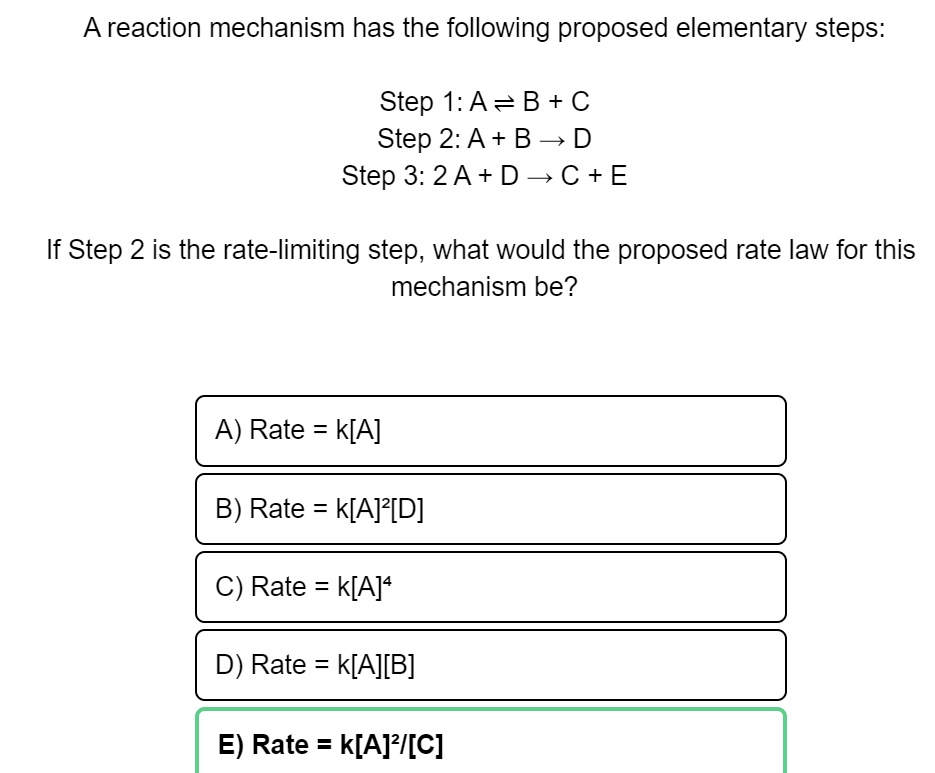
SOLVED:A reaction mechanism has the following proposed elementary steps: Step 1:A= B + € Step 2: A+ B~ D Step 3: 2A+ D_C+E If Step 2 is the rate-limiting step, what would

SOLVED:17. Two mechanisms are proposed: H2O2 +1 v H2o+OI OI- +Ht _ HOI HOI+I-+Ht _ Iz+ Hzo I2 +I - I3" H2Oz + I + Ht + H2o +HOI HOI+I-+Ht-Iz + HzO
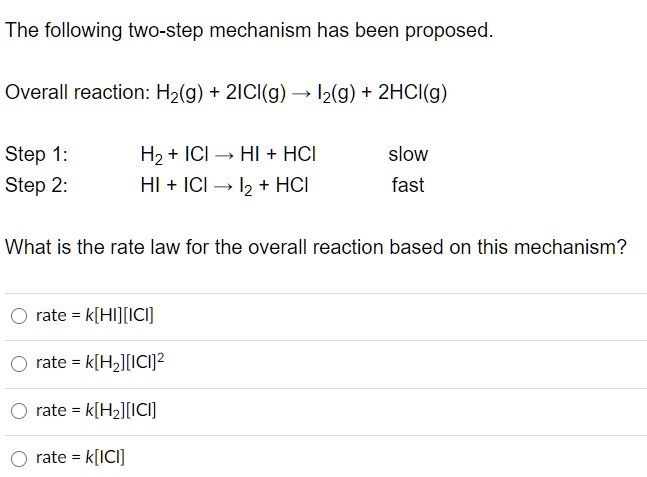
SOLVED:The following two-step mechanism has been proposed: Overall reaction: Hz(g) + 2ICI(g) Iz(g) 2HCI(g) Step 1: Step 2= Hz + ICI ~, HI HCI HI + ICl I2 + HCI slow fast