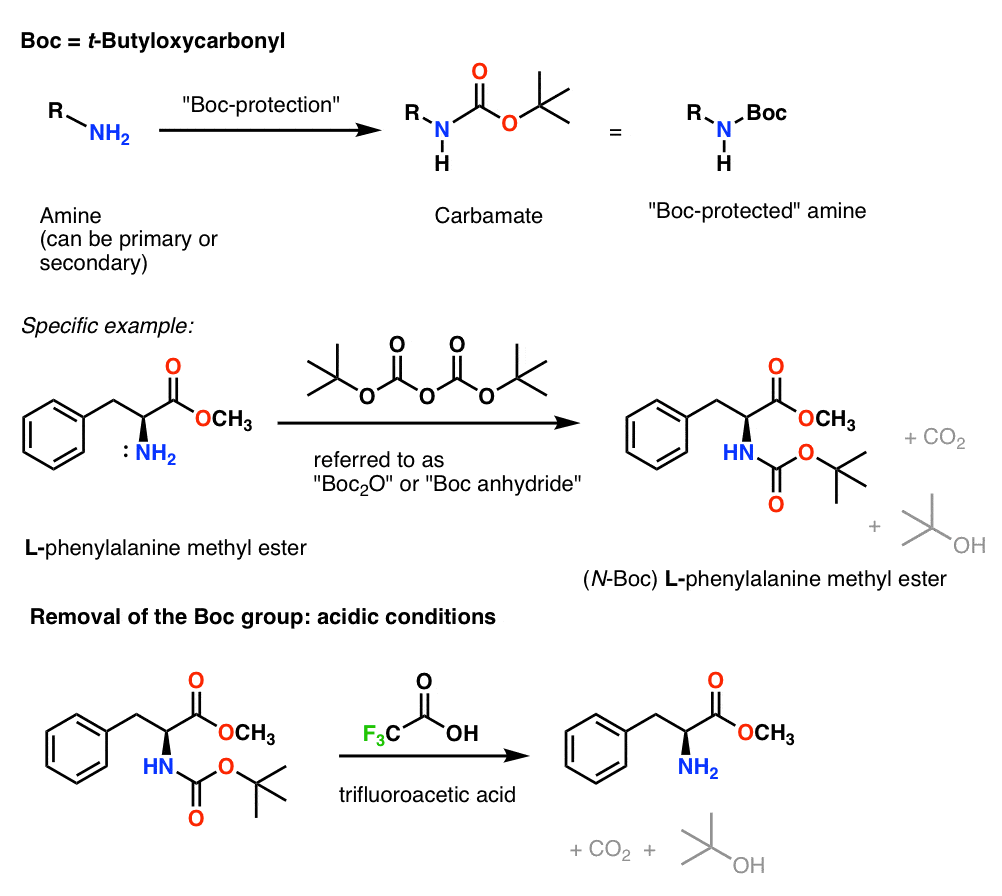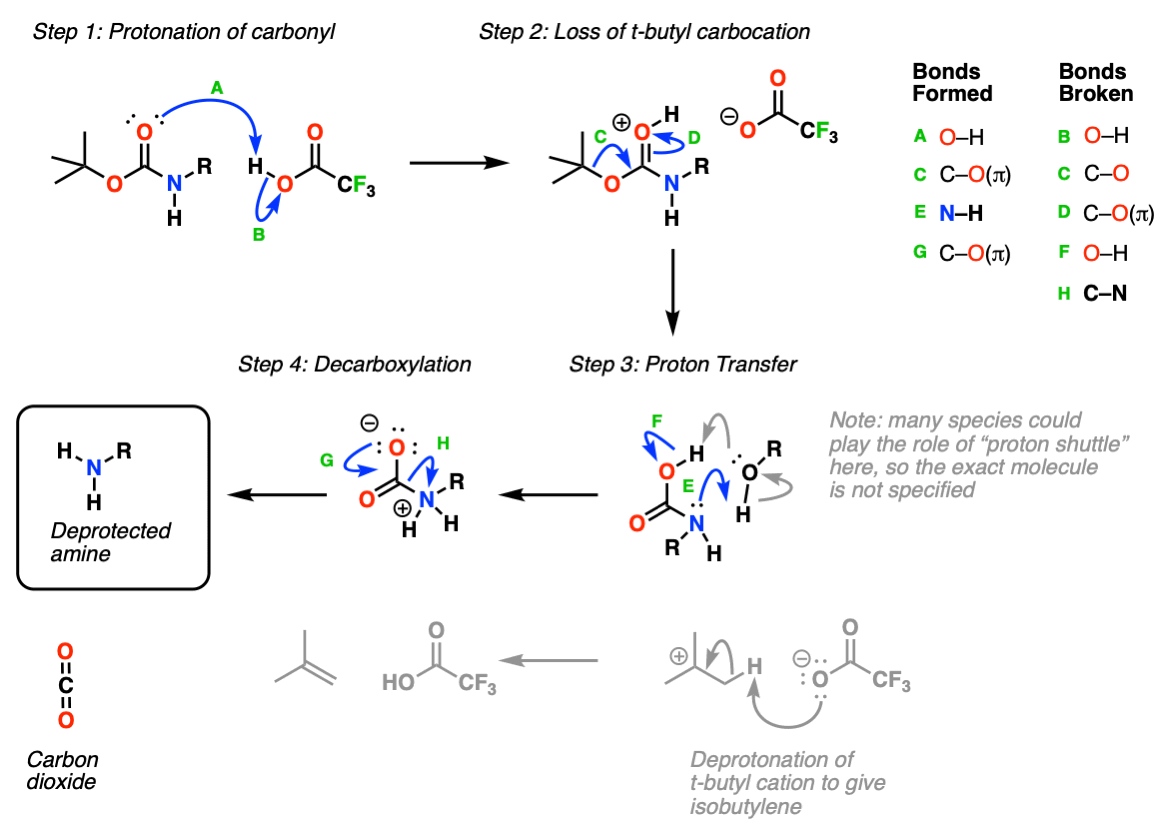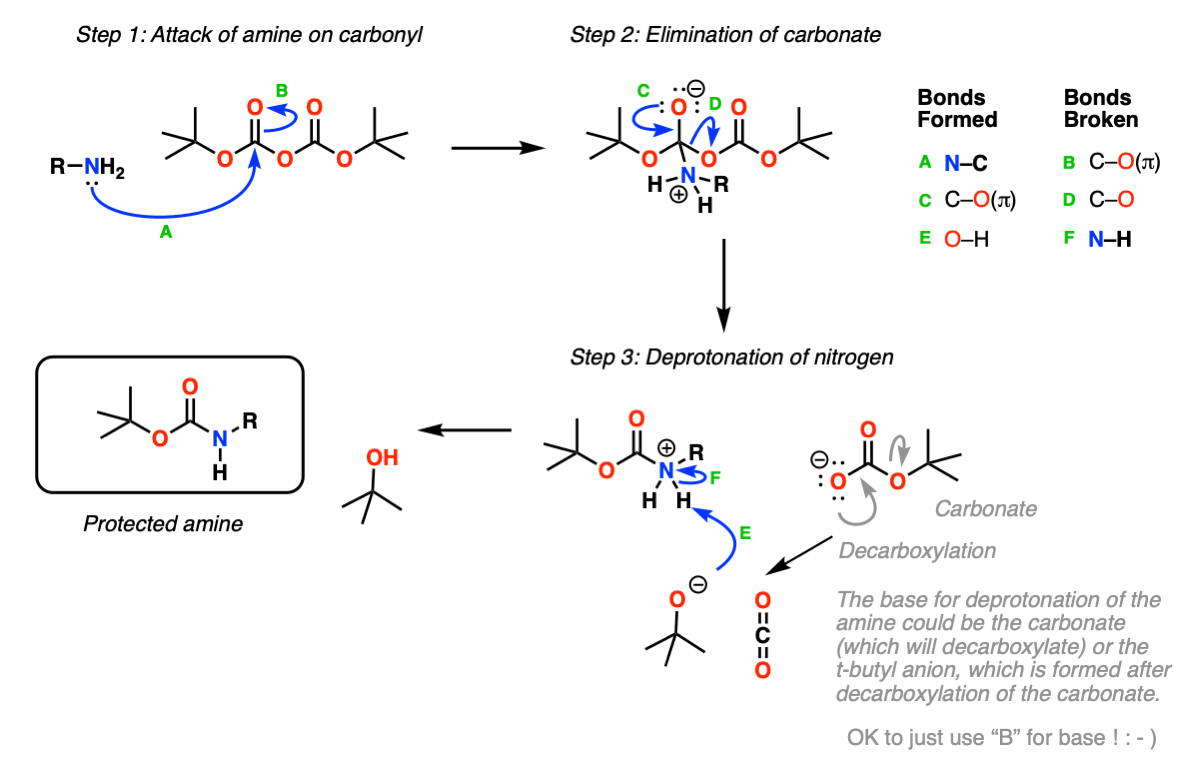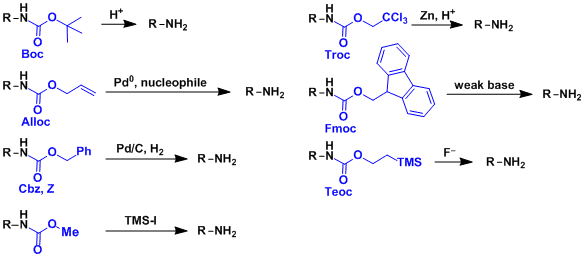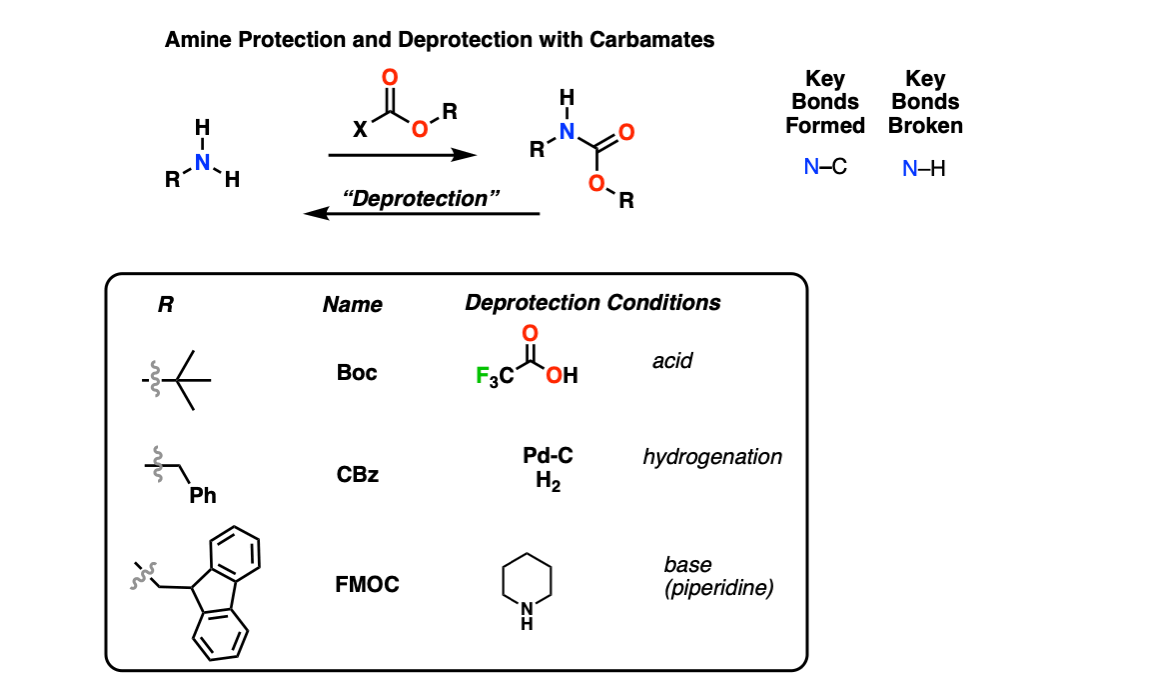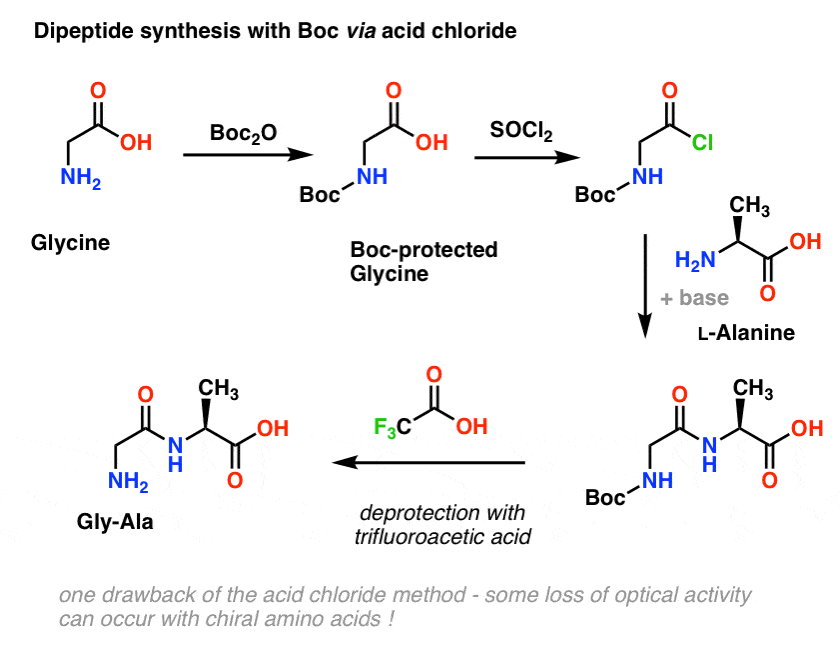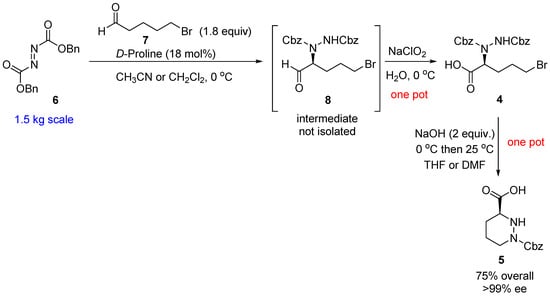
Molecules | Free Full-Text | A Reliable Enantioselective Route to Mono-Protected N1-Cbz Piperazic Acid Building Block | HTML

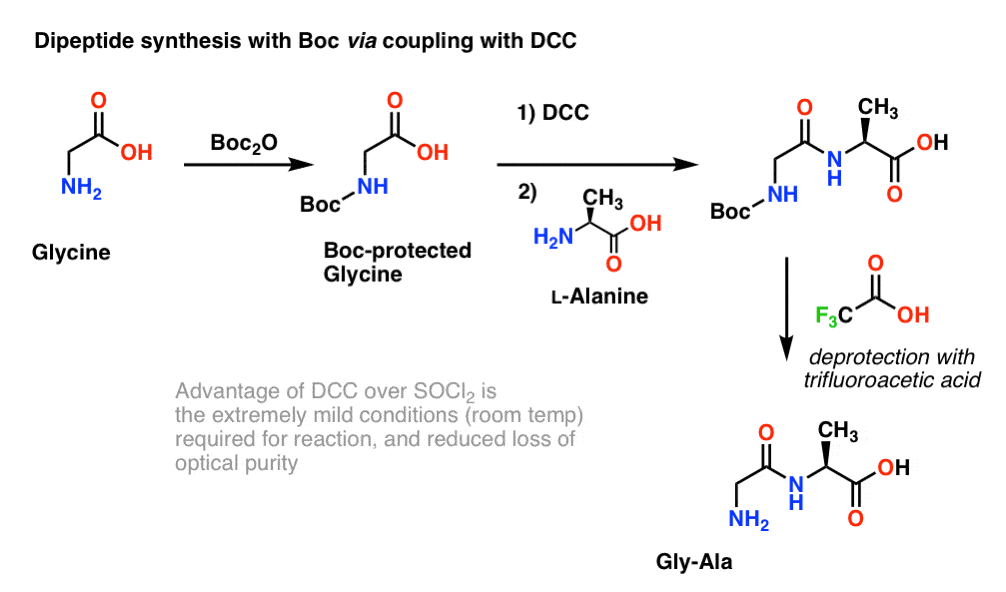
Tertiary-butoxycarbonyl (Boc) – A strategic group for N-protection/ deprotection in the synthesis of various natural/unnatural N-unprotected aminoacid cyanomethyl esters - ScienceDirect

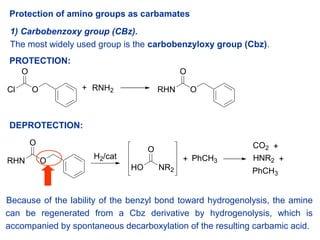
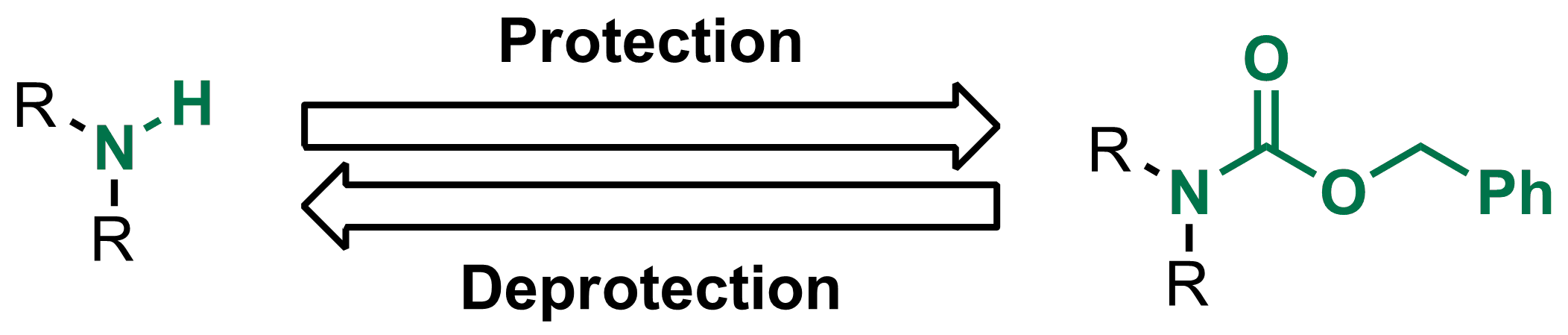
A convenient protocol for the deprotection of N-benzyloxycarbonyl (Cbz) and benzyl ester groups - ScienceDirect
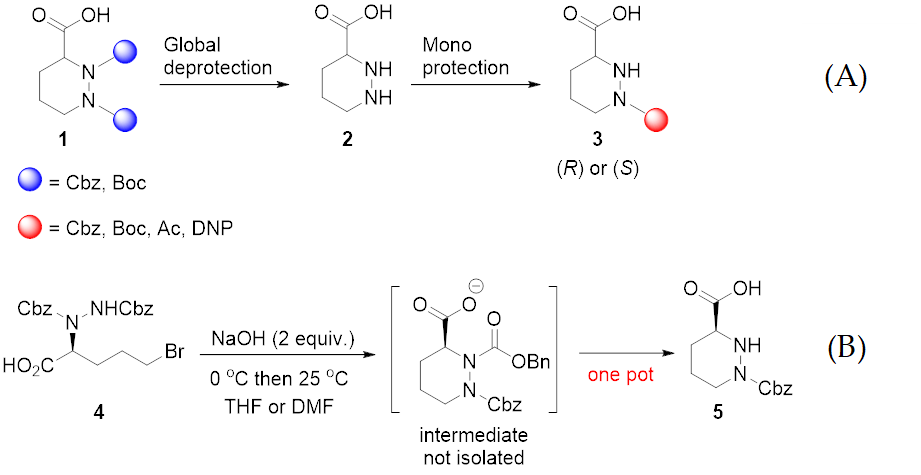
Molecules | Free Full-Text | A Reliable Enantioselective Route to Mono-Protected N1-Cbz Piperazic Acid Building Block | HTML
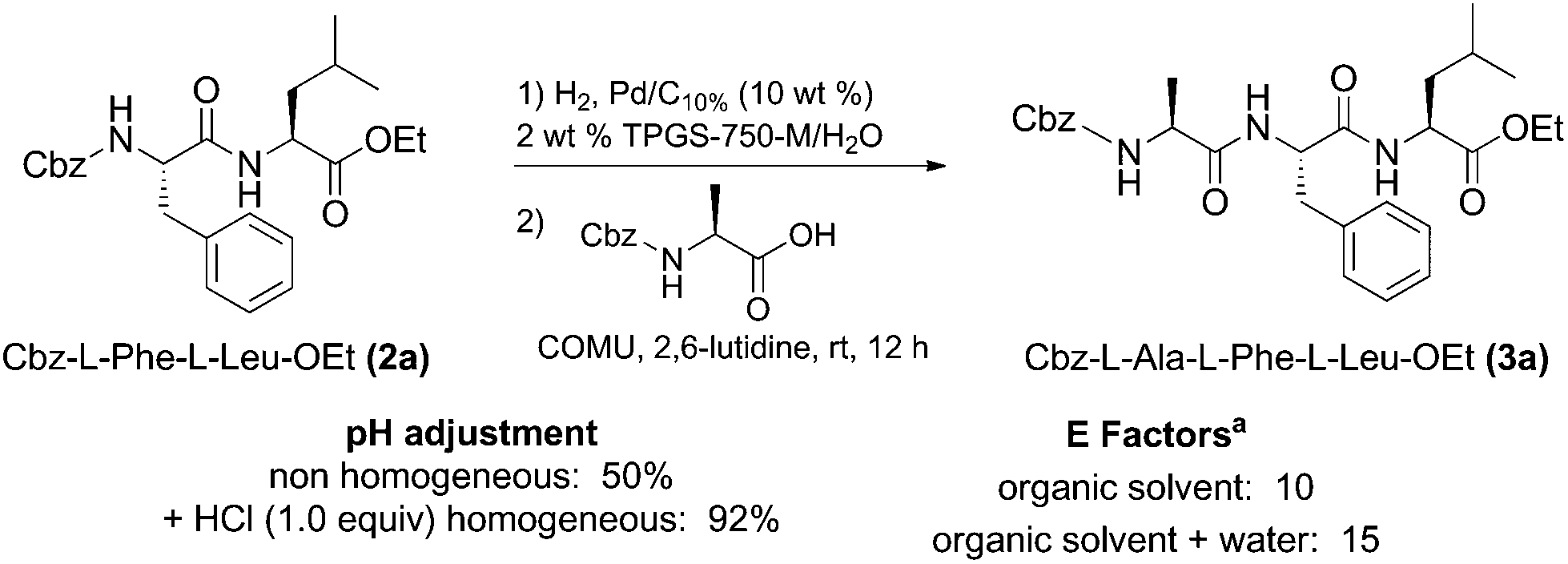
Tandem deprotection/coupling for peptide synthesis in water at room temperature - Green Chemistry (RSC Publishing) DOI:10.1039/C7GC01575E

organic chemistry - Deprotection of carboxybenzyl (Cbz) in the presence of Methyl ester? - Chemistry Stack Exchange

Selective deprotection of the Cbz amine protecting group for the facile synthesis of kanamycin A dimers linked at N-3″ position - ScienceDirect